copper electron configuration full|What is the electron configuration of cop : Manila To write the configuration for the Copper ions, first we need to write the electron configuration for just Copper (Cu). We first need to find the number of .
Check out free Hot Romantic Sex porn videos on xHamster. Watch all Hot Romantic Sex XXX vids right now!
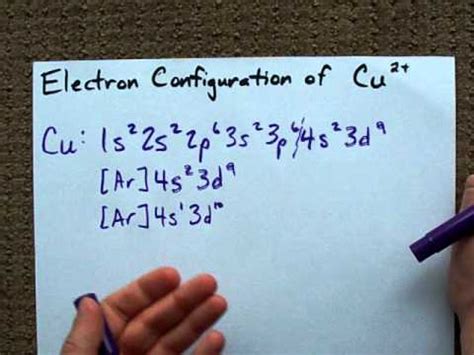
copper electron configuration full,How to Write the Electron Configuration for Copper (Cu, Cu+, and Cu2+) In order to write the Copper electron configuration we first need to know the number of electrons for the Cu atom (there are 29 electrons). Once we have the configuration for Cu, the ions are simple. Mar 23, 2023 Copper is a chemical element of the periodic table with chemical symbol Cu and atomic number 29 with an atomic weight of 63.5463 u and is classed as transition metal and is . Full Electron Configuration For Copper. The full electron configuration for copper is [Ar] 3d10 4s1.copper electron configuration full Causey shows you step by step how to write the electron configuration and orbital notation for copper (Cu). You will learn about the orbital overlapping and the . To write the configuration for the Copper ions, first we need to write the electron configuration for just Copper (Cu). We first need to find the number of . The electron configuration of copper (Cu) includes a fully-filled 3d subshell. Cu: 1s 2 2s 2 2p 6 3 s 2 3p 6 4s 1 3d 10. The actual electron configuration of .Key Takeaways. Copper’s electron configuration is 1s²2s²2p⁶3s²3p⁶4s¹3d¹⁰, deviating from the standard electron filling order due to the stability gained from a half-filled or fully-filled .What is the electron configuration of cop This electron configuration calculator will instantly show you the distribution of electrons in the orbitals of any periodic element you choose. Typically, .
copper electron configuration full What is the electron configuration of cop This electron configuration calculator will instantly show you the distribution of electrons in the orbitals of any periodic element you choose. Typically, .Electron atomic and molecular orbitals A Bohr diagram of lithium. In atomic physics and quantum chemistry, the electron configuration is the distribution of electrons of an atom or molecule (or other physical structure) in atomic or molecular orbitals. [1] For example, the electron configuration of the neon atom is 1s 2 2s 2 2p 6, meaning that the 1s, 2s, and .Key Takeaways. Copper’s electron configuration is 1s²2s²2p⁶3s²3p⁶4s¹3d¹⁰, deviating from the standard electron filling order due to the stability gained .
If you need to write the full electron configuration for an anion, then you are just adding additional electrons and the configuration is simply continued. . In the d block, specifically the groups containing Chromium .
The electron configurations of silicon (14 electrons), phosphorus (15 electrons), sulfur (16 electrons), chlorine (17 electrons), and argon (18 electrons) are analogous in the electron configurations of their outer shells to their corresponding family members carbon, nitrogen, oxygen, fluorine, and neon, respectively, except that the principal quantum .

Similarly, the observed electron configuration of copper is [Ar] 4s 1 3d 10 instead of [Ar] s 2 3d 9. The actual electron configuration may be rationalized in terms of an added stability associated with a half-filled (ns 1, np 3, nd 5, nf 7) or filled (ns 2, np 6, nd 10, nf 14) subshell. Given the small differences between higher energy levels .

Writing Electron configuration of Copper. Mr. Causey shows you step by step how to write the electron configuration and orbital notation for copper (Cu). You. The electron configuration of copper (Cu) includes a fully-filled 3d subshell. Cu: 1s 2 2s 2 2p 6 3 s 2 3p 6 4s 1 3d 10. The actual electron configuration of these elements may be rationalized in terms of an added stability associated with a half-filled (ns 1, np 3, nd 5, nf 7) or filled (ns 2, np 6, nd 10, nf 14) subshell. 3. Continue the electron configuration from the noble gas until you reach the element of interest. 4. Put the noble gas in brackets and write the remainder of the electron configuration. Na has the same electron configuration as Ne with the addition of 3s 1. Na's noble gas configuration is [Ne]3s 1.
What is the electron configuration of copper? The electron configuration of copper (Cu) is 1s² 2s² 2p⁶ 3s² 3p⁶ 4s¹ 3d¹⁰. . Copper’s electron configuration is unique because it has a full 3d subshell with 10 electrons, even though the 4s subshell is not full. This is due to the stability gained by having a half-filled or fully .The electron configurations of silicon (14 electrons), phosphorus (15 electrons), sulfur (16 electrons), chlorine (17 electrons), and argon (18 electrons) are analogous in the electron configurations of their outer shells to their corresponding family members carbon, nitrogen, oxygen, fluorine, and neon, respectively, except that the principal .Electronic configuration of Copper: The atomic number of Copper(Cu) = 29; Therefore, the expected electronic configuration is Ar 3 d 9 4 s 2. But Copper has an exception in electronic configuration due to the stability concept of orbitals, completely filled and half filled orbitals are the most stable. Thus, the observed electronic of copper is .Electron configuration for copper. Electron configuration. . Full configuration: 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 1: Electron configuration chart. 1s 2: 2s 2: 2p 6: 3s 2: 3p 6: 3d 10: 4s 1: Electrons per shell: 2, 8, 18, 1: Valence electrons : 1: Valency electrons : 1,2: Bohr model: Electron shell for Copper, created by Injosoft AB Cu.
Element Copper (Cu), Group 11, Atomic Number 29, d-block, Mass 63.546. Sources, facts, uses, scarcity (SRI), podcasts, alchemical symbols, videos and images. . Electron configuration The arrangements of electrons above the last (closed shell) noble gas. Melting point The temperature at which the solid–liquid phase change occurs. The electronic configuration of copper (Cu), with an atomic number of 29, is 1s² 2s² 2p⁶ 3s² 3p⁶ 4s¹ 3d¹⁰. This unique configuration is characterized by one electron in the 4s orbital and ten electrons in the 3d orbital, .
The valence electrons (here 3s 2 3p 3) are written explicitly for all atoms. Electron configurations of elements beyond hassium (element 108) have never been measured; predictions are used below. As an approximate rule, electron configurations are given by the Aufbau principle and the Madelung rule.
Hydrogen has its only electron in the 1s orbital - 1s 1, and at helium the first level is completely full - 1s 2. The second period. Now we need to start filling the second level, and hence start the second period. Lithium's electron goes into the 2s orbital because that has a lower energy than the 2p orbitals.
Similarly, the observed electron configuration of copper is [Ar]4s 1 3d 10 instead of [Ar]s 2 3d 9. The actual electron configuration may be rationalized in terms of an added stability associated with a half-filled (ns 1, np 3, nd 5, nf 7) or filled (ns 2, np 6, nd 10, nf 14) subshell. Given the small differences between higher energy levels .
copper and chromium. This order of occupation roughly represents the increasing energy level of the orbitals. Hence, electrons occupy the orbitals in such a way that the energy is kept at a minimum. . Hence the full or expanded electronic configuration for bromine in accord with the Aufbau Principle is 1s 2 2s 2 2p 6 3s 2 3p 6 .
copper electron configuration full|What is the electron configuration of cop
PH0 · Writing the Electron Configuration for Copper (Cu)
PH1 · What is the electron configuration of cop
PH2 · Electron Configuration for Cu, Cu+, and Cu2+ (Copper and
PH3 · Electron Configuration for Cu, Cu+, and Cu2+ (Copper and
PH4 · Electron Configuration for Copper (Cu, Cu+, Cu2+)
PH5 · Electron Configuration for Copper (Cu, Cu+, Cu2+)
PH6 · Electron Configuration for Copper (Cu,
PH7 · Electron Configuration For Copper
PH8 · Electron Configuration Chart of All Elements (Full Chart)
PH9 · Electron Configuration Calculator
PH10 · Copper Electron Configuration (Cu) with Orbital Diagram
PH11 · Copper Electron Configuration (Cu) with
PH12 · Copper (Cu)
PH13 · 1.9: Electron Configurations for Transition Metal Elements